
About a year ago I was invited to co-author a chapter on the geochemistry of pore water for the upcoming edition of Treatise on Geochemistry. Working with two incredible colleagues, Pei-Chuan Chuang from the National Central University in Taiwan and Andrea Erhardt from the University of Kentucky we spent summer 2023 writing and checking in with each other regularly on Zoom. The best part? Having the extra nudge to catch up on some recent papers and making figures, I really enjoy tweaking figures to make them just like we had envisioned. The hardest part of the process? I would have to say managing the scope of the chapter. The number of times I would be starting on one topic and get momentarily distracted by an ‘oh that’s cool.. should we add a section on…?” and constant temptation to deep dive down another worm hole… I’m not going to say we were the best ‘no’ support group, but we did ok keeping checks on each other to maintain reasonable bounds on the chapter.

The aim of the chapter is to provide a resource for those interested in pore water, and also be pitched at such a level that it could help a graduate student (or upper level undergraduate) really start to dive into the pore water world. We wanted the figures to be both useful for teaching and presentation intros etc.So what did we end up including? We focused on three main themes, 1) controls on pore water geochemistry (what processes are happening and how the environment makes a difference), 2) what pore water and concentration profiles of pore water can tell us about the system and any sediment alteration, and 3) current methods for collecting and processing porewater.
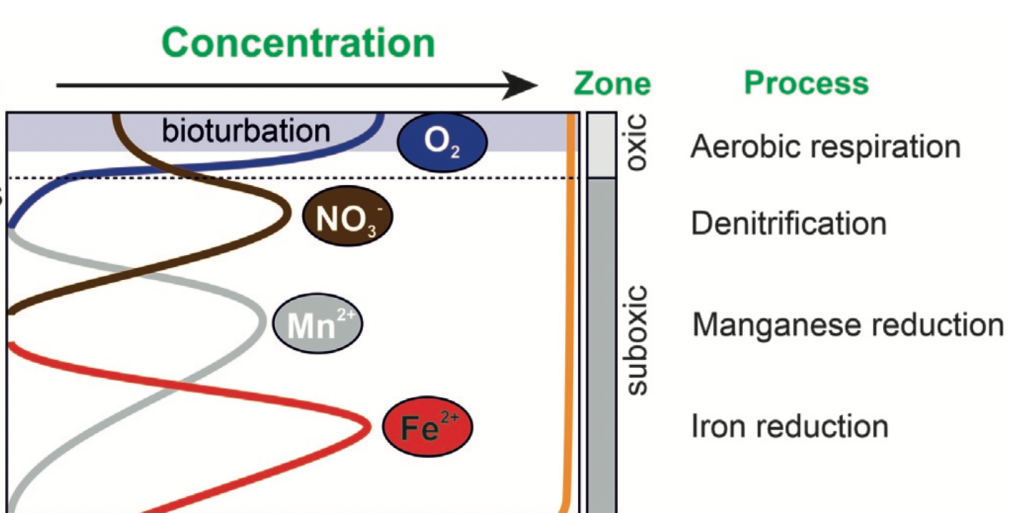
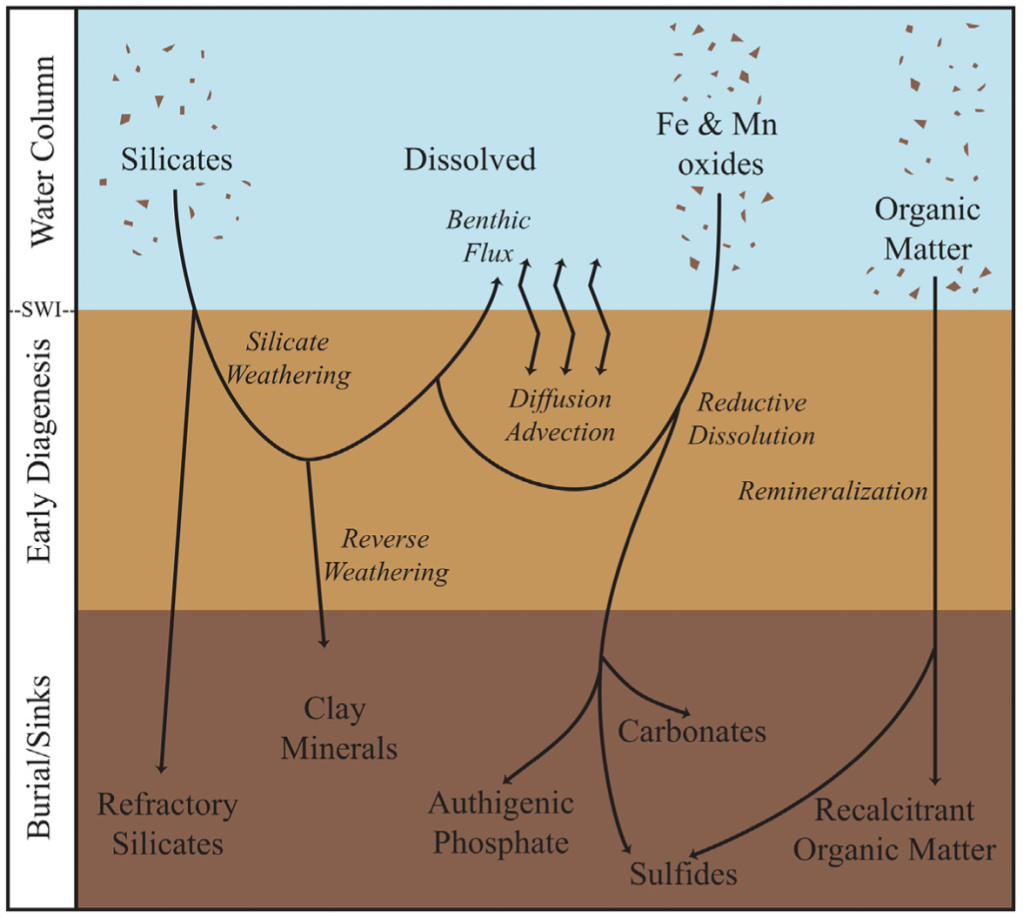
As you might expect, especially if you’ve taken a college level chemistry class, redox plays a dominant role in controlling the chemistry and is therefore featured front and center in the chapter. If you’re not a chemist, think of redox as the presence/absence of oxygen (and if that is enough for you, go ahead and skip down to the next paragraph). Redox, short for REDuction and OXidation reactions, essentially looks at where the energy in the system is coming from (via the transfer of electrons). If there is oxygen present, the most energetically favorable (most energy for least input) comes from aerobic respiration, the same process you and I do. When there isn’t free oxygen present, for a bit more work energy can be obtained through the removal of oxygen from other molecules – e.g. removing oxygen from iron oxide through ‘iron reduction.’ Because the ultimate goal is energy, these reactions change in a predictable manner from the most energetically favorable towards the sediment water interface moving down to less and less favorable the deeper into the sediments you go, thus earning this specific series the name ‘redox ladder’ or ‘redox cascade.’
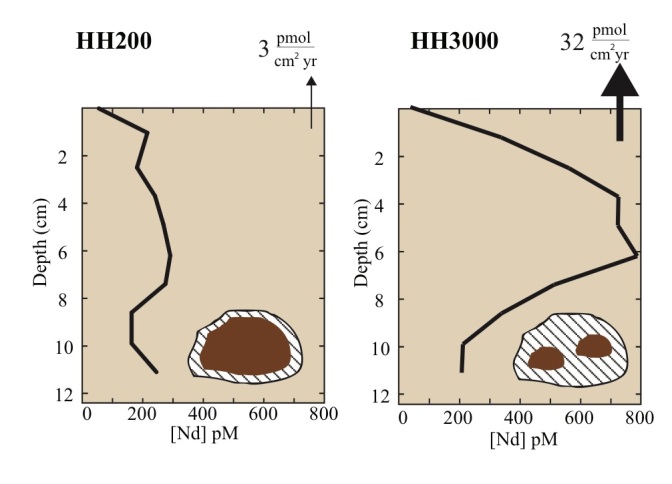
Beyond oxygen as a controlling variable, other factors such as pH, grain size, and how pore water and its constituents are transported are also important. We go through a brief overview of diffusion (slow, just the element moves and not the water) versus advection (fast, the water and everything it contains moves together), and look at the role of biology and key abiotic factors in determining the dominant transport regime. Of course, different elements are going to be impacted differently so we break down these controlling geochemical processes by element or groups of interest (e.g. nitrogen cycle, silicates, methane, carbonates, and trace elements and their isotopes). We also explore the role of the pore water as a sink, source, or both of each category of elements to the overlying ocean water.
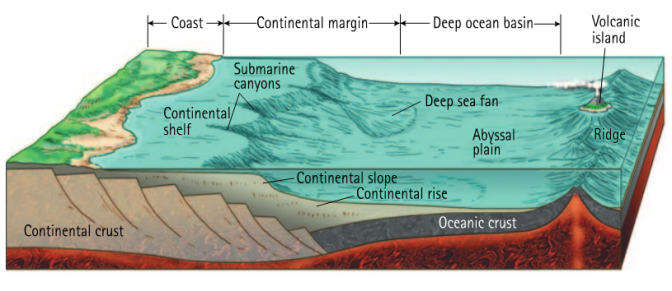
We compare behavior in the major marine environments (shelf, slope, abyssal, hadal, upwelling zones, and restricted basins), often linking each environment to key physical constraints or sediment properties that add to its ‘typical’ pore water behavior. Our discussion isn’t limited to the modern ocean, with discussion of how dominant geochemical reactions would have been similar or different in the past and how that understanding informs the use of sediment proxies in reconstructing past ocean environments and ocean-climate interactions through time.
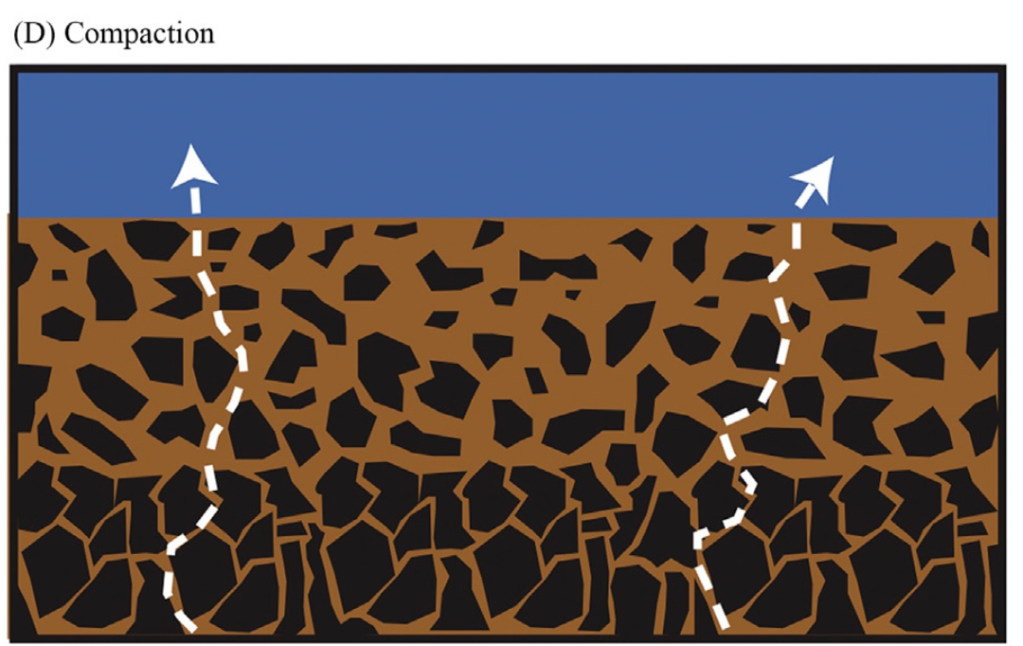
Method wise, we give a brief overview of the most commonly used techniques as well as emerging state-of-the-art techniques including pros, cons, and which measurements they are most appropriate for (if known!). We try to address how much volume is needed for each measurement, potential artifacts, known yield or blank issues, and general feasibility. Towards the end, we even include a brief section on modelling pore water geochemistry and current computational methods.
Want to know more? Check out the full chapter online now, and as always if you are paywalled/can’t access it please contact me.
Also of interest might be:
Neodymium and grain size Abbott et al 2016 (early observations) or Abbott et al 2019 (more clay specific).