First: where is part 1? Well, I started a manuscript title with ‘Bottoms up’ back in 2015 when we looked at the isotopic influence of a benthic flux of neodymium out of the sediments (the first pore water neodymium isotope measurements!) and while I won’t go into detail here you can check out the paper here and you can check out a blog post on a later part of that project at Out of the Mud. Similar story for part 2, but a different element! Meet Chromium…

Back in 2018, I was alternate-chief scientist and sediment-lead on a research cruise aboard RV Investigator with a group of geochemists from around the world. You may have read about that voyage here (see Science in The Swells). One of those scientists on board was Dave Janssen, and as is one of the major benefits of research voyages with several groups on board – a new collaboration started. Since then, Dave has added the pore water from that voyage to multiple other efforts to better understand the ocean’s chromium cycle. The result was recently published in Earth and Planetary Science Letters (It is open access! That means everyone can read the paper for free!).

The paper has a little bit of everything – release from biogenic particles, benthic fluxes, and ocean circulation so I highly recommend checking out the full thing if you’re interested. But first, why chromium? You’re probably most familiar with chromium as a trace nutrient that you get from a variety of foods and even some types of beer or for its role in producing stainless steel. In the ocean, chromium has shown promise in being able to tell us about how oxygen levels have changed through time because in the modern ocean, redox conditions exert control over both dissolved chromium concentrations and the stable isotope composition of chromium.
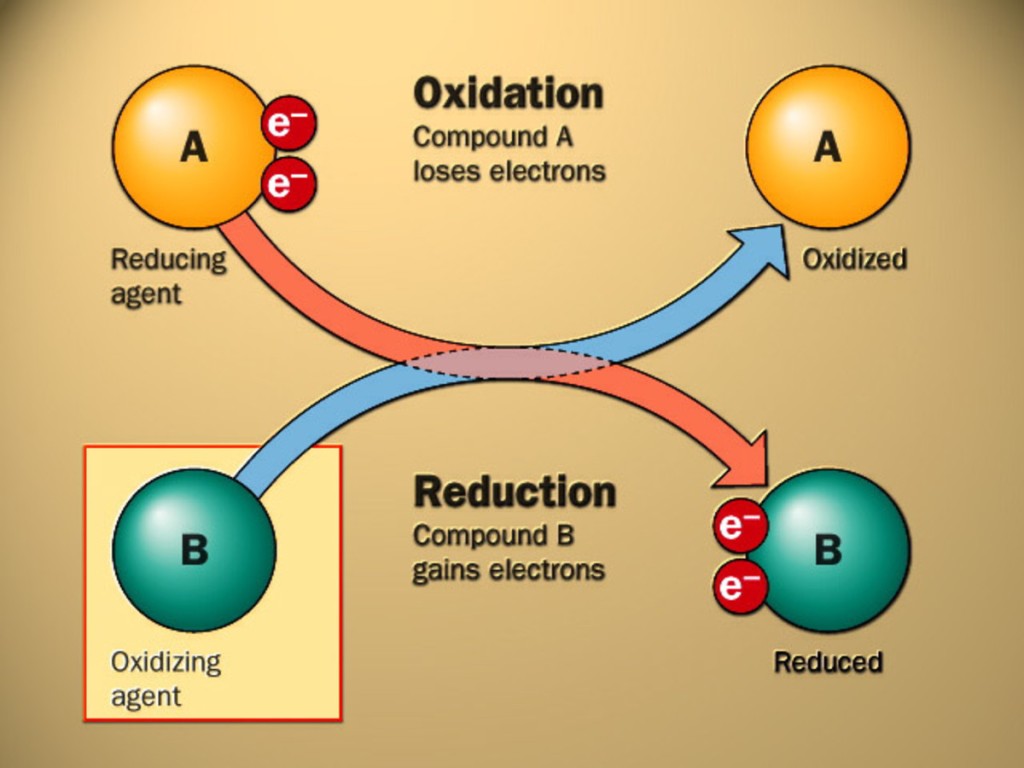
Ok- some of the chemistry basics (simplified and very brief). Chromium has two dominant oxidation states in the ocean, Cr3 and Cr6 (remember way back to Chemistry 101- oxidation state has to do with the loss or gain of electrons which ultimately controls the charge of the ion. Oxygen is a major ‘oxidizing agent’ which gains electrons during these reactions- ‘B’ in the schematic). The important part here is that these two forms of Cr behave very differently. Cr6 is relatively soluble and doesn’t react to particles whereas Cr3 (‘the reduced form’) is not soluble (it doesn’t readily dissolve in water) and is strongly attracted to particles (it will adsorb onto anything it possibly can rather than floating around freely). This difference in behavior means the main sink for dissolved chromium in the ocean is through particle scavenging in oxygen minimum zones (OMZs). This means that the Chromium sink is : 1) spatially limited, and relies on the 2) biologically mediated export of 3) reduced chromium. These same redox transformations (switching between Cr6 and Cr3) largely happening in OMZs cause isotopic fractionation with Cr reduction associated with lighter isotopes. Because this demonstrates the isotopes of Cr are being driven by the availability of oxygen, we should be able to use the isotopes from the sediment record to reconstruct past oxygen levels. But reliably using Cr as a ‘proxy’ for ocean oxygenation requires understanding the interaction of processes that control Cr budgets (both concentration and isotopes) in the ocean and during incorporation into the sediment record.
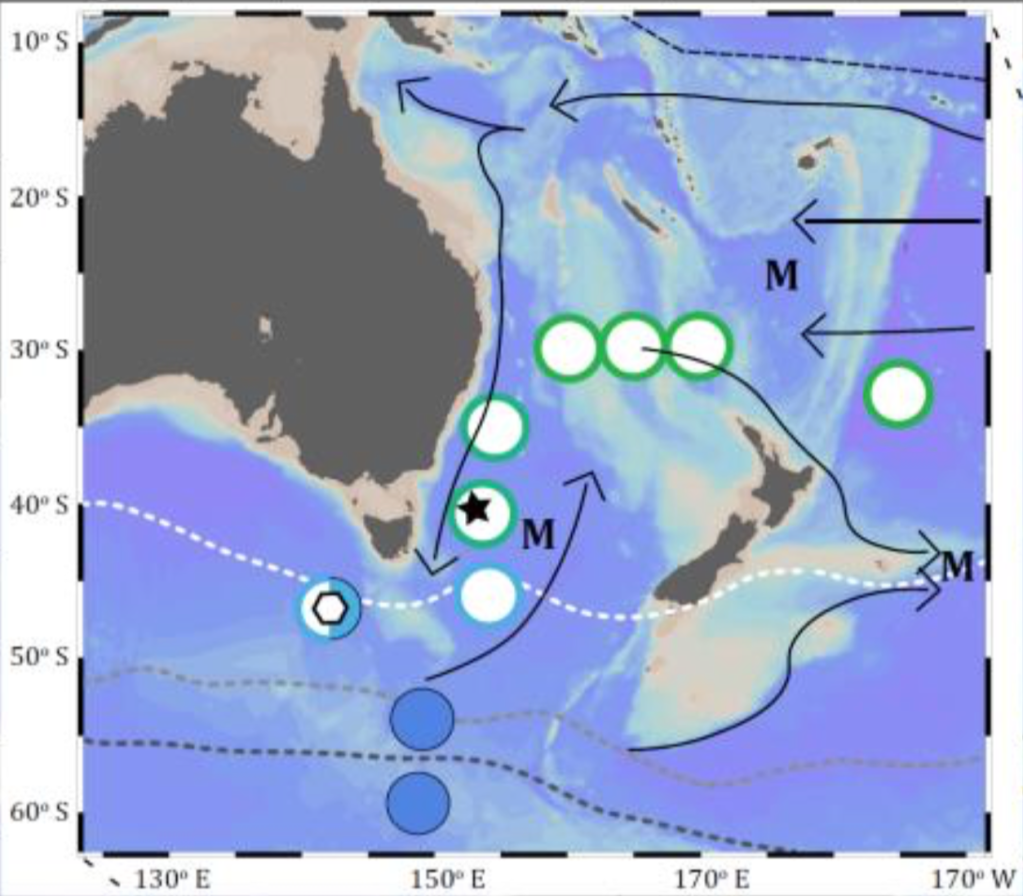
Here, I’m going to focus on the benthic flux part of the chromium story that I was most involved in (I discuss a similar story for Nd in the Tasman from 2016 in Out of the Mud). Elevated Cr concentrations in bottom water have been well documented since the 1980s, consistent with a bottom (benthic) source of chromium to the overlying water. Off the California shelf, this bottom water enrichment has been attributed to release of Cr from decomposing organic matter (respiration – just like what we do! We break down organic matter using oxygen and give off CO2).
Ok, so what did we find in the Tasman? Pore water was enriched in dissolved Cr (about 1 order of magnitude greater than measured in bottom water). While the concentration of Cr in the porewater decreased with depth in the sediment column it was still elevated (slightly) relative to bottom water at its lowest observed point. Interestingly, our maximum Cr concentration was right near the sediment water interface – this creates a sharp concentration gradient, suggesting pore waters could act as a source of dissolved Cr to the bottom water through a diffusive flux. Doing the calculations, if these calcareous sediments are representative of global oxic sediments (still a big IF, we need more samples!) then the benthic source of Cr from sedimentary pore waters to the ocean would be comparable to (or even bigger than) the amount of Cr coming from rivers (rivers are currently assumed to be the biggest Cr source to the ocean).
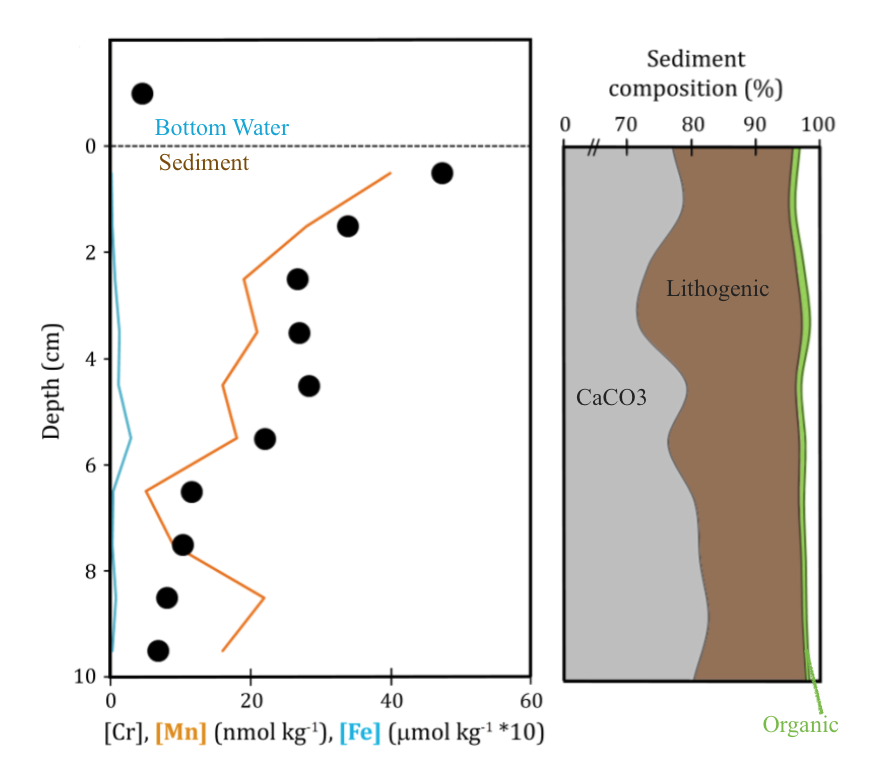
What drives this benthic source of Cr is a bit of a more complicated story. If we use the sediment composition (major contributors) and the reported Cr concentrations of those sediment components in the upper sediment column, we find that none of the major phases (calcium carbonate, lithogenic, or phytoplankton) can account for the Cr flux. But several mechanisms that can result in Cr enrichment in sinking particles could make up the difference (e.g. decoupling of Cr from organic matter during respiration, scavenging of Cr3). Regardless of which mechanism, these enrichment processes largely rely on the Cr ultimately originating in the water column and then being incorporated into the sediments through scavenging. In which case the benthic flux may act more as a moderator of the sink rather than as an entirely new source. But again, we need more mud! Similar data from diverse sediment types is needed to determine whether the lithogenic material is contributing ‘new’ Cr to the pore water and the potential influence of minor or trace phases.

As I said, there is A LOT in this paper – far too much to go into here, but I do want to mention our major conclusions (even from the sections I didn’t dive into here):
- Chromium release from sinking biogenic particles (e.g. dead plankton) is independent from the release of major elements (Cr is decoupled from carbon, nitrogen, phosphorous); this Cr release may be driven by oxidation (potentially MnOx).
- Benthic Cr fluxes are certainly important locally, and may be a crucial component of the global Cr budget for the ocean — in the calcareous sediment examined, this flux is likely derived from water column derived Cr that had been scavenged onto particles and sank to the sediment column rather than newly released Cr from lithogenic particles.
- Biogenic export from the surface ocean and subsequent re-release or Cr at depth (either in the bottom water or via a benthic flux) is important in shaping the distribution of Cr concentration and Cr isotope distributions in the ocean
For more, check out the paper at EPSL!
Full Citation:
Janssen D.J., Rickli J., Abbott A.N., Ellwood M.J., Twining B.S., Ohnemus D.C., Nasemann P., Gilliard D., and Jaccard S.L. (2021) Release from biogenic particles, benthic fluxes, and deep water circulation control Cr and δ⁵³Cr distributions in the ocean interior, Earth and Planetary Science Letters, 574, doi: 10.1016/j.epsl.2021.117163

2 thoughts on “Bottoms up: part 2”